Home / Pipeline / Parkinson’s Drug
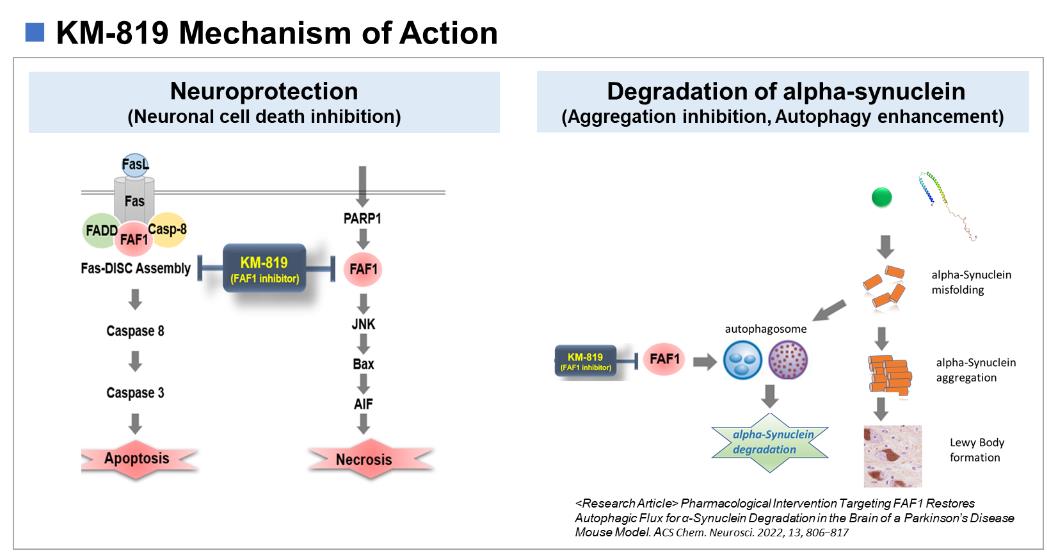
KM-819, unlike the current approaches to the treatment of Parkinson’s disease (PD), is an innovative new drug based on the new mode of action that protects dopaminergic neurons from death by inhibiting the function of FAF1 which is known to induce cell death. KM-819 showed the efficacy of neuroprotection and inhibiting behavioral impairment induced by MPTP treatment in animals.
At present, there are four classes of therapy for PD, Dopamine supplement therapy (Levodopa), Dopaminergic agonist (Ropinirole), COMT inhibitor (Tolcapone) and MAO-B inhibitor (Selegiline).At present, there are four classes of therapy for PD, Dopamine supplement therapy (Levodopa), Dopaminergic agonist (Ropinirole), COMT inhibitor (Tolcapone) and MAO-B inhibitor (Selegiline).
The pharmacological function of these therapies is limited to relieving PD symptoms but with various complications leading to serious side effects such as insomnia, movement impairment and tremors
There has been no drugs available which slow or halt the progression of the disease until our drug KM-819 was discovered. Thus, we hope that KM-819 would be the first-in-class disease modifying therapeutic for Parkinson’s disease.
Currently KM-819 has completed its Phase I clinical study in Korea, and we aim to prove its effectiveness on Parkinson's disease through Phase II clinical study.